DURHAM, N.C. -- Every great exploration refines the map, and in the case of the massive BRAIN initiative funded by the National Institutes of Health, that new draft of the map has just been released.
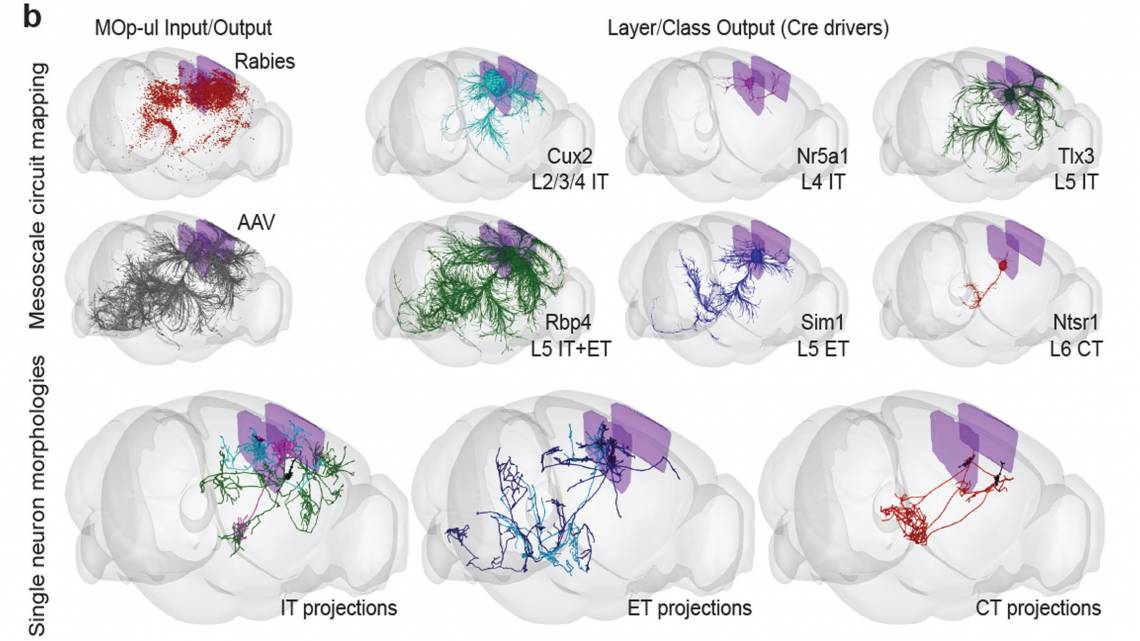
In a special collection of 17 new papers appearing Wednesday in the journal Nature, an international consortium of more than 250 neuroscientists is describing in unprecedented detail the structure – cell by cell -- of the motor cortex in the mouse, marmoset monkey, and human brains. The effort has also produced a comprehensive cellular-level wiring diagram of the mouse primary motor cortex.
Duke’s Josh Huang, professor of neurobiology, has been one of the ring-leaders of the four-year effort. Huang is one of 13 corresponding authors and one of three principal manuscript editors on the flagship paper that describes the contents of 16 projects, and is also lead author on two other articles of the package.
Huang said comparisons might be drawn to the decade-long effort to sequence the human genome for the first time, but there are some important differences. “When the genome project started, we knew what a gene was and we knew how to sequence it,” he said. “But in brain science, we still have a lot of debate on what is a neuron type and we are continuing to understand deeper what that is.” And the brain, unlike the genome, is not a linear sequence, he points out.
“The definition and biological basis of neuron types in the brain remain contentious,” he said. “While genes and genomes are linear nucleotide sequences and DNA sequencing technology was well established, neurons and their connectomes are high-dimensional and multimodal, thus the brain challenge requires developing a range of new technologies,” Huang said.
The researchers behind the BRAIN Initiative Cell Census Network (BICCN) have examined millions of neurons and other kinds of cells in the motor cortex and sorted them into hierarchical categories, which may comprise several major classes, dozens of major types and perhaps more than 100 subtypes. In all, the brain has about 160 billion cells creating trillions of critical connections.
So, as massive as this new atlas is, it’s only the beginning, Huang said. “It's the primary motor cortex. It's not the whole cortex, it's certainly not the whole brain. Those are the next steps.”
“To understand how a system works, you need to first build a parts list,” said Hongkui Zeng, Ph.D., Executive Vice President and Director of the Allen Institute for Brain Science, a division of the Allen Institute which led several BRAIN Initiative-funded studies. “Then you have to understand what each part is doing and put the pieces together to understand how the whole system works. That’s what we’re doing with the brain.”
The mapping so far is characterizing each of the different kinds of cells in the motor cortex and estimating how many of each type there are and where they are located. And then their connectivity, the complexity that drives the brain’s abilities, is also starting to be mapped.
Connectivity is very difficult to see and map, “because the shape (of a neuron) is so large and so small at the same time,” Huang said.
Huang, who has developed many genetic engineering tools to study the brain’s circuitry, did much of the work on the new motor cortex atlas at Cold Spring Harbor Laboratory before he joined Duke as a Science and Technology Initiative faulty member in 2020. He will continue the mapping work here and wants to grow it to encompass more Duke faculty.
In that effort, Huang will be helped in part by a new NIH Director’s Pioneer Award, announced Tuesday. The grant of up to $700,000 per year for five years is part of the High-Risk, High-Reward Research program, which supports scientists developing pioneering approaches to major challenges. Huang will be using the support to develop a new generation of precise and programmable cell engineering technologies to monitor and edit the function of diverse cell types across animal organs and species. “This may have broad applications in biomedical research, biotechnology, and therapeutics.,” he said.
Creators of the motor cortex atlas believe their techniques will now pave the way for mapping the entire mammalian brain. The end goal is more than just navigation, however. For example, a useful map of the motor cortex should also lead to better understanding of brain diseases that attack the neurons that control movement, like amyotrophic lateral sclerosis, or ALS.
Many of the insights in the new papers comes from single cell transcriptomics, Huang said. That’s a relatively new and very powerful technology that allows researchers to measure the molecular genetic contents of individual cells. Using a tool called spatial transcriptomics, the researchers were able to map the precise position of individual cell types defined by their mRNA expression profiles, and register these in 3D brain space.
“We’re not quite there yet, but the goal is to register not only the positions of cell bodies, but also chart their connections,” Huang said.
“In my own lab, we would like to use this information to achieve a deeper understanding of neural development and function across levels of brain organization,” Huang said. “To do that, one of the key steps is to use this molecular information and turn it into genetic tools that can identify and manipulate these different cell types.”
CITATION: “A Multimodal Cell Census and Atlas of the Mammalian Primary Motor Cortex,” Nature, Oct. 6, 2021.