The Making of a Root
How plants harness 'bad' molecules for good ends

DURHAM, N.C. -- When most people think of a plant, they picture stems, leaves, flowers, and all the parts that are visible above ground. But Duke biologist Philip Benfey is more interested in the hidden half of the plant that is buried beneath the soil. Roots: they may be out of sight, Benfey says, but they play critical roles, anchoring the plant and taking up water and nutrients.
Now, Benfey and colleagues Masashi Yamada and Xinwei Han have pieced together new details in the cascade of events that guide root growth -- research that could lead to more productive crops optimized for different soil types.
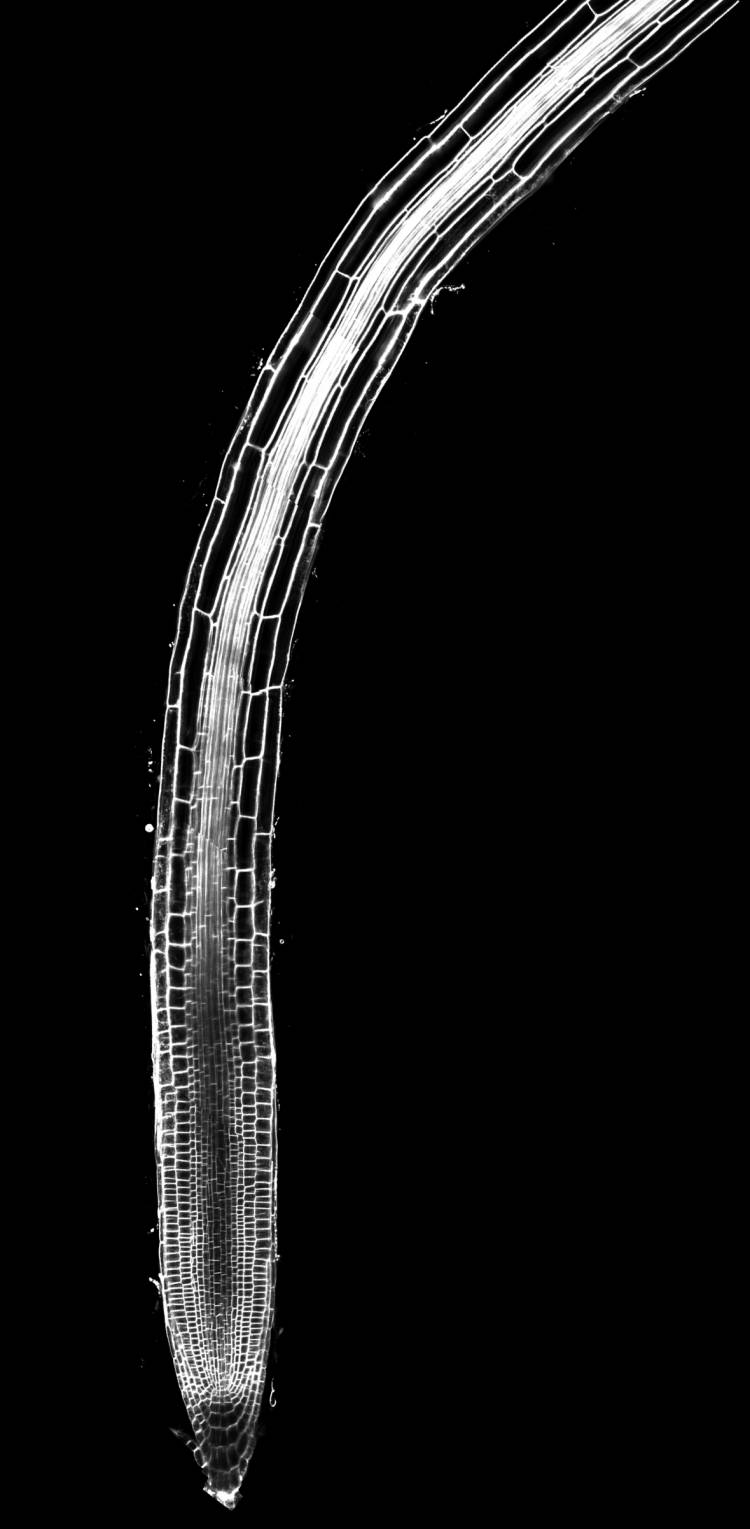 As a root tunnels through the soil, stem cells in the root’s tip must determine whether to divide and produce more of the same stem cells, or differentiate into other cell types, based on their location within the root tissue. In a study published in the journal Nature, the researchers show that cells get some of the information they need from substances that are usually thought to be harmful.
As a root tunnels through the soil, stem cells in the root’s tip must determine whether to divide and produce more of the same stem cells, or differentiate into other cell types, based on their location within the root tissue. In a study published in the journal Nature, the researchers show that cells get some of the information they need from substances that are usually thought to be harmful.
Natural byproducts of cellular respiration, molecules called “reactive oxygen species” have long been described as stress signals that can cause tissue damage if left unchecked. But they also play a role in cell signaling, Benfey’s work shows.
In a study of the small flowering plant Arabidopsis thaliana, the researchers report that root growth is partly regulated by interactions between two types of reactive oxygen species, superoxide and hydrogen peroxide, as they build up in different regions of the root tip.
“What we did was map out, from signal to response, how these supposedly toxic chemicals are harnessed for a signaling process,” Benfey said.
Roots grow longer thanks to a small region of stem cells at the end of each root that produces a constant supply of new cells behind it, propelling the root tip further downward through the soil like the head of a bullet. The daughter cells that are left behind stay put, and eventually stop dividing and start to specialize.
How fast a root grows depends on the balance between two opposing cues: those that encourage these stem cells to keep multiplying, and those that tell them to put the brakes on proliferating and change gears to specialize. The researchers identified a protein called RITF1 that, when activated, triggers this developmental switch.
The protein works by controlling where the two reactive oxygen species concentrate within the growing tip of the root.
These chemical signals tell the surrounding cells what course of action to take next. Cells exposed to higher amounts of superoxide keep dividing and producing new cells, while those that get a heavy dose of hydrogen peroxide differentiate, with a zone of transition where the two overlap.
“We don’t have all the pieces yet,” Benfey said, “but there are a lot more steps of the process that are now known through this work than were known before.”
“Reactive oxygen species aren’t just toxic chemicals,” Benfey said. “They serve important roles as regulators of a developmental process, going from a stem cell to fully differentiated tissue.”
This research was supported by the Howard Hughes Medical Institute, the Gordon and Betty Moore Foundation (GBMF3405), and the U.S. National Institutes of Health (MIRA 1R35GM131725).
CITATION: "RGF1 Controls Root Meristem Size Through ROS Signalling," Masashi Yamada, Xinwei Han, Philip N. Benfey. Nature, December 4, 2019. DOI: 10.1038/s41586-019-1819-6