
DURHAM, N.C. -- A high-fat meal can silence communication between the intestine and the rest of the body, according to a new Duke University study in zebrafish.
While using the fish to examine cells that normally tell the brain and the rest of the body what’s going on inside the gut after a meal, a team of Duke researchers discovered that a high-fat meal completely shuts down that communication for a few hours.
The cells they were looking at are the enteroendocrine cells, which occur sparsely throughout the lining of the gut, but play a key role in signaling the body about the all-important alimentary canal. In addition to releasing hormones, the cells also have a recently-discovered direct connection to the nervous system and the brain.
These cells produce at least 15 different hormones to send signals to the rest of the body about gut movement, feelings of fullness, digestion, nutrient absorption, insulin sensitivity and energy storage.
“But they fall asleep on the job for a few hours after a high-fat meal, and we don’t yet know if that’s good or bad,” said John Rawls, an associate professor of molecular genetics and microbiology in the Duke School of Medicine.
Since enteroendocrine cells are key players in digestion, the feeling of being full and subsequent feeding behavior, this silencing may be a mechanism that somehow causes people eating a high-fat diet to eat even more.
“This is a previously unappreciated part of the postprandial (after-meal) cycle,” Rawls said. “If this happens every time we eat an unhealthy, high-fat meal, it might cause a change in insulin signaling, which could in turn contribute to the development of insulin resistance and Type 2 diabetes.”
To understand the silencing better, the researchers tried to break the process down step by step in zebrafish.
After they first sense a meal, the enteroendocrine cells trigger a calcium burst within seconds, initiating the signaling process. But after that initial signal there’s a delayed effect later in the after-meal period. It’s during this later response that the silencing occurs, said Rawls, who also directs Duke’s Microbiome Center.
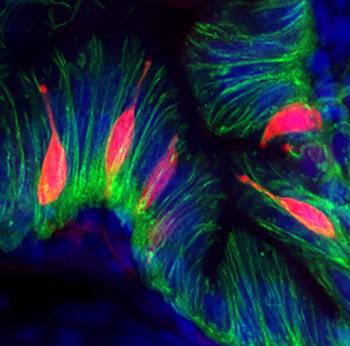
The silenced cells change shape and experience stress in their endoplasmic reticulum, a structure that assembles new proteins. It seems that these enteroendocrine cells, which are specialized to synthesize and secrete proteins like hormones and neurotransmitters, become overstimulated and exhausted for a while.
The team tried the high-fat diet on a line of germ-free zebrafish raised in the absence of any microbes, and found they didn’t experience the same silencing effect. So they began looking for gut microbes that might be involved in the process.
After screening through all the kinds of bacteria found in the gut, they saw that the silencing appeared to be the work of a single type of gut bacteria, called Acinetobacter. These bugs are normally less than 0.1 percent of the total gut microbiome, but they increased 100-fold after a high-fat meal and were the only bacteria able to induce the silencing effect.
“Next we want to understand how Acinetobacter evokes this interesting response,” said Lihua Ye, a postdoctoral fellow and lead author on this paper. “We also suspect other bacteria might also have this capability.”
Rawls said they aren’t sure why silencing occurs, nor whether it has any positive effect on the fish. It might be a way to prevent excessive signaling about the fat, but by being silenced completely like this, the cells won’t be communicating anything else either.
“We don’t understand yet what the long-term impact of enteroendocrine silencing would be on metabolic health,” Rawls said. “This may be a maladaptive response to high-fat feeding that impairs the normal regulatory functions of these cells, leading to metabolic disorders like insulin resistance. But it’s also possible that the silencing is a beneficial adaptation to protect the animal from over-stimulation of the gut cells.”
The study appeared as an accepted manuscript at the open-access journal eLife on Dec. 3. This research was funded by the National Institute of Diabetes and Digestive and Kidney Disease (R01-DK093399, R01 DK109368, R01-DK081426) and the Pew Charitable Trusts.
CITATION: “High-Fat Diet Induces Microbiota-Dependent Silencing of Enteroendocrine Cells,” Lihua Ye, Olaf Mueller, Jennifer Bagwell, Michel Bagnat, Rodger Liddle, John Rawls. eLife, Dec. 3, 2019. DOI: 10.7554/eLife.48479