Blood pressure drugs, like many medications in use today, often have ‘off-target’ effects because we don’t yet understand exactly how they work.
New research out of Duke, UCLA, Stanford and Harvard is showing precisely how the crucial cell surface receptors interact differently with various drugs, giving the researchers hope that they may be able to tailor more specific medications for heart patients.
High blood pressure affects one in three American adults, increasing the risk of heart disease and stroke for about 75 million Americans.
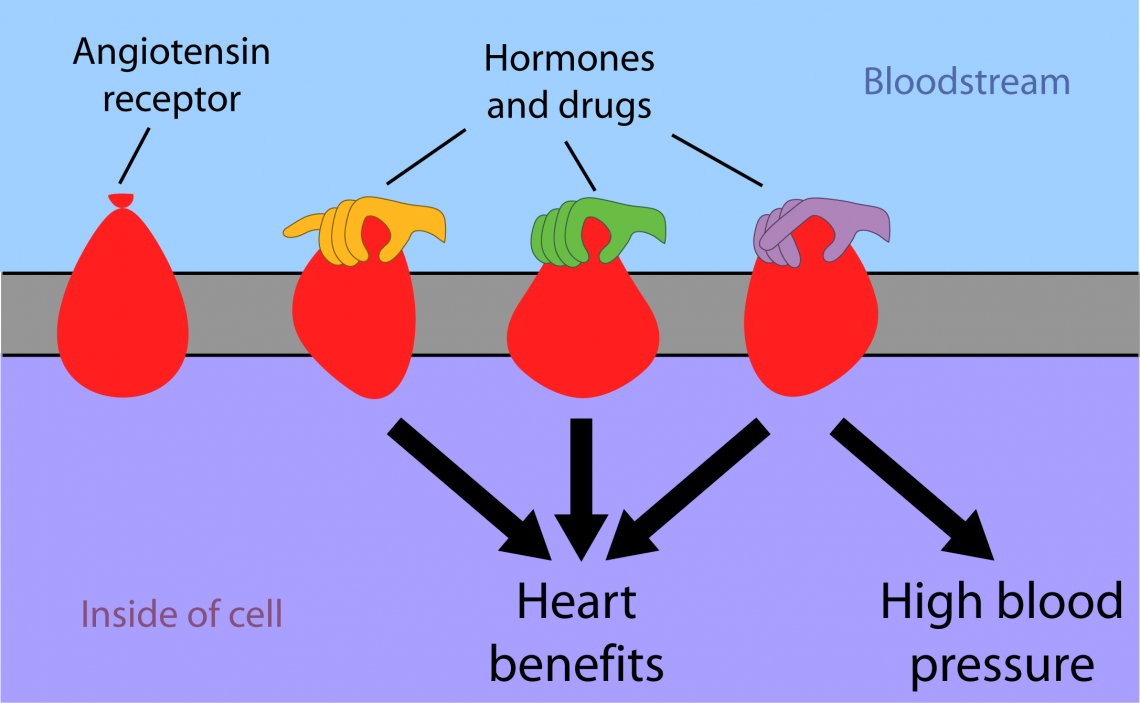
The blood vessel constriction which raises blood pressure is triggered by the interaction of a hormone, angiotensin II, with the angiotensin receptor on the surface of cells in the heart, blood vessels, kidney, adrenal cortex, lungs and brain. Blood pressure drugs called angiotensin receptor blockers (ARBs) treat high blood pressure by preventing angiotensin II from binding to its receptor.
But in doing so, these drugs also block angiotensin II’s beneficial effects, including increases in the heart’s strength and performance.
Ideally, physicians would like to block the angiotensin receptor’s effects on blood pressure without losing its positive effects on heart function, said cardiologist Robert Lefkowitz, M.D., the James B. Duke professor of Medicine and senior author of one of two companion papers coming out in Cell on Jan. 10.
Duke researchers collaborated with scientists across the country to determine the various shapes the angiotensin receptor assumes when it is turned on by different types of drugs, a key step towards being able to design better heart medicines.
“For a long time, people assumed that these receptors had one ‘off state’ and one ‘on state,’ like a light switch,” said lead author Laura Wingler, Ph.D., a postdoctoral researcher in the Lefkowitz laboratory. “But they aren’t light switches; these receptors are more like dials with multiple settings, or states. What’s been unclear for the past 10 years is what these receptor states look like and why each state triggers different events inside the cell.”
Just as squeezing only the top of a balloon alters its entire shape, the binding of hormones and drugs to the outside of a receptor causes changes in parts of the receptor which face into the cell. Different hormones and drugs push different “buttons” on a receptor, changing its shape in different ways.
Seeing those specific shapes of the angiotensin receptor for the first time “helps us approach the design of drugs more rationally,” Wingler said. “Now we know what to be aiming for and the mechanisms we need to target.”
“Millions of people take drugs that turn this receptor off, and it has been studied very thoroughly, but because of technological limitations, people couldn’t see what it looks like when it’s turned on,” said Andrew Kruse, associate professor of biological chemistry and molecular pharmacology at Harvard Medical School and co-senior author of the paper.
The Duke-led study, which included colleagues at UCLA and Stanford, used a sophisticated technique called double electron-electron resonance spectroscopy to map the shape of the receptor when it interacts with different classes of hormones and drugs.
“It’s like seeing a silhouette of the receptor -- an outline of its shape from one viewpoint,” Wingler said.
The researchers discovered that the receptor assumes four main shapes: one associated with ARBs that turn the receptor completely off, one associated with angiotensin II and drugs that turn the receptor fully on (both increasing blood pressure and improving heart function), and two associated with the drugs that improve heart function without increasing blood pressure.
In a second paper, the Duke group worked with the laboratory of Andrew C. Kruse, a professor at Harvard Medical School, to use X-ray crystallography to see the fine details of the receptor when it is frozen in the “fully on” state.
Wingler compares this approach to seeing an intricate, three-dimensional statue of the receptor. Importantly, it let them see how one drug interacts with the receptor and what “buttons” it presses to change the shape of the receptor.
The angiotensin receptor is a member of a family of proteins called G protein-coupled receptors (GPCRs), which sit in the membrane that surrounds cells and interact with hormones and drugs in the bloodstream. The GPCR family includes receptors for adrenaline, histamine, opioids, and the many molecules responsible for taste and smell, and they are the target of about one-third of all FDA-approved drugs.
Lefkowitz and Stanford University Professor Brian Kobilka, a senior co-author on one of the Cell papers, shared the 2012 Nobel Prize in Chemistry for discovering the GPCR family and defining how these receptors work.
The researchers hope these latest findings may lead to tailor-made drugs for other GPCRs that could separate desired therapeutic effects from unwanted side effects.
For example, these same principles have already been used to develop new drugs for the opioid receptor that have advanced to clinical trials. These next-generation opioid receptor drugs relieve pain but are less prone to cause the side effects associated with morphine and fentanyl, such as constipation and potentially lethal slowed breathing.
“Our papers go way beyond anything which has been done in this field before,” Lefkowitz said. “This research is likely to lead to discovery and development of novel types of drugs which can manipulate these receptors’ shapes in ways that have not previously been possible.”
“Our study provides a framework for interpreting a lot of data that’s been collected over the years about the receptor’s function, helping to turn disparate pieces into a single coherent model,” Kruse said.
The research was partially funded by National Institutes of Health grants (RO1HL016037), the Mandel Center for Hypertension and Atherosclerosis at Duke and the Howard Hughes Medical Institute.
CITATIONS: “Angiotensin analogs with divergent bias stabilize distinct receptor conformations,” Laura M. Wingler, Matthias Elgeti, Daniel Hilger, Naomi R. Latorraca, Michael T. Lerch, Dean P. Staus, Ron O. Dror, Brian K. Kobilka, Wayne L. Hubbell, Robert J. Lefkowitz, Cell, Jan. 10, 2019. DOI: 10.1016/j.cell.2018.12.005
“Distinctive Activation Mechanism for Angiotensin Receptor Revealed by a Synthetic Nanobody,” Laura M. Wingler, Conor McMahon, Dean P. Staus, Robert J. Lefkowitz, Andrew C. Kruse. Cell, Jan. 10, 2019. DOI: 10.1016/j.cell.2018.12.006