Duke researchers have discovered a unique repair mechanism in the developing backbone of zebrafish that could give insight into why spinal discs of longer-lived organisms like humans degenerate with age.
The repair mechanism apparently protects the fluid-filled cells of the notochord, the precursor of the spine, from mechanical stress as a young fish begins swimming. Notochord cells go on to form the gelatinous center of intervertebral discs, the flat, round cushions wedged between each vertebrae that act as shock absorbers for the spine.
The disappearance of these cells over time is associated with degenerative disc disease, a major cause of human pain and disability worldwide.
“It is not difficult to speculate that these same mechanisms of repair and regeneration are present in humans at very early stages, but are lost over time," said Michel Bagnat, Ph.D., senior author of the study and assistant professor of cell biology at Duke University School of Medicine. “If we are going to think about techniques that foster intervertebral disc regeneration, this is the basic biology we need to understand.”
The study appears June 22, 2017, in Current Biology.
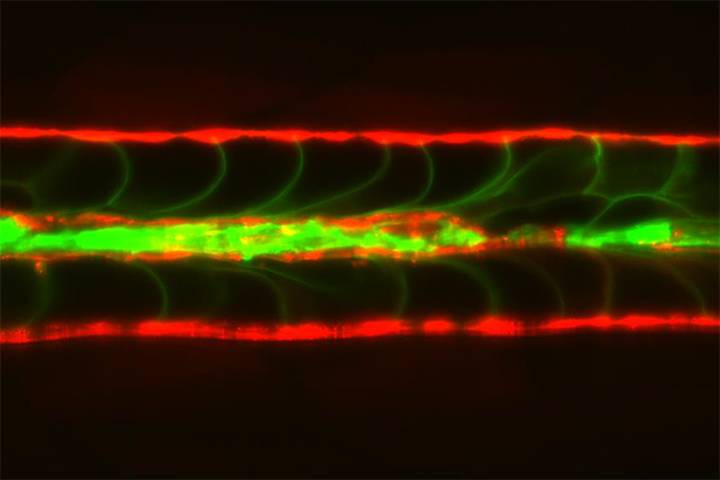
Bagnat likens the notochord to a garden hose filled with water. The hardy structure consists of a sheath of epithelial cells surrounding a collection of giant fluid-filled or “vacuolated” cells. During development, these vacuolated cells rarely pop, despite being under constant mechanical stress. Recent research has suggested that tiny pouches known as caveolae (Latin for “little caves”) that form in the plasma membrane of these cells can provide a buffer against stretching or swelling.
To see whether the caveolae protected vacuoles from bursting, his team and collaborators from Germany generated mutants of three caveolar genes in their model organism, the zebrafish. Because these small aquarium fish are transparent as embryos, the scientists could easily visualize any spinal defects triggered by the loss of caveolae.
The researchers found that when the mutant embryos hatched and started swimming, exerting pressure on their underdeveloped backbones, their vacuolated cells started to break up. While the finding confirmed their suspicions, it turned up a puzzling discovery. “In the caveolar mutants, you see these serial lesions up and down the notochord, and yet the mature spine formed normally,” said Bagnat. “That was very puzzling to us.”
To figure out how that was possible, lead authors Jamie Garcia and Jennifer Bagwell took a closer look at the notochord of mutant fish. They marked the vacuolated cells green and the surrounding epithelial sheath cells red and then filmed the fish shortly after they hatched and started swimming. First, they could see an occasional vacuolated cell break and spill its contents like a water balloon. Then, over the course of fifteen hours, a nearby epithelial sheath cell would move in, crawl over the detritus of the collapsed cell, and morph into a new vacuolated cell.
They performed a few more experiments and found that the repair response was triggered by the release of the cell contents, specifically the basic molecular building blocks known as nucleotides. The researchers then isolated live epithelial sheath cells and treated them with nucleotide analogs to show that they turned into vacuolated cells.
“These cells, which reside in the discs of both zebrafish and man, seem capable of controlling their own repair and regeneration,” said Bagnat. “Perhaps it is a continuous release of nucleotides that is important for keeping the disc in good shape.”
The study may offer insight not only into the development of back and neck pain, but also into the origins of cancer. Their data suggests that chordomas, rare and aggressive notochord cell tumors, may begin when epithelial sheath cells leave the notochord and invade the skull and other tissues.
The research was supported by National Institutes of Health (AR065439, AR065439-04S1, T32DK007568-26, and CA193256), a Capes-Humboldt Fellowship, the Max Planck Society, and a Faculty Scholar grant from the Howard Hughes Medical Institute.
CITATION: "Sheath cell invasion and trans-differentiation repair mechanical damage caused by loss of caveolae in the zebrafish notochord," Jamie Garcia, Jennifer Bagwell, Brian Njaine, James Norman, Daniel S. Levic, Susan Wopat, Sara E. Miller, Xiaojing Liu, Jason W. Locasale, Didier Y.R. Stainier and Michel Bagnat. Current Biology, June 22, 2017. DOI# 10.1016/j.cub.2017.05.035