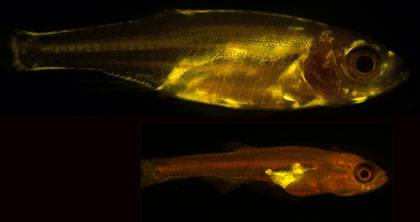
Scientists have known for some time that people who carry a lot of weight around their bellies are more likely to develop diabetes and heart disease than those who have bigger hips and thighs. But what hasn’t been clear is why fat accumulates in different places to produce these classic “apple” and “pear” shapes.Now, researchers have discovered that a gene called Plexin D1 appears to control both where fat is stored and how fat cells are shaped, known factors in health and the risk of future disease.Acting on a pattern that emerged in an earlier study of waist-to-hip ratios in 224,000 people, the study, which appears March 23 in the Proceedings of the National Academy of Sciences, found that zebrafish that were missing the Plexin D1 gene had less abdominal or visceral fat, the kind that lends some humans a characteristic apple shape. The researchers also showed that these mutant zebrafish were protected from insulin resistance, a precursor of diabetes, even after eating a high-fat diet.“This work identifies a new molecular pathway that determines how fat is stored in the body, and as a result, affects overall metabolic health," said John F. Rawls, Ph.D., senior author of the study and associate professor of molecular genetics and microbiology at Duke University School of Medicine. “Moving forward, the components of that pathway can become potential targets to address the dangers associated with visceral fat accumulation.”Unlike the subcutaneous fat that sits beneath the skin of the hips, thighs, and rear of pear-shaped individuals, visceral fat lies deep within the midsection, wedged between vital organs like the heart, liver, intestine, and lungs. From there, the tissue emits hormones and other chemicals that cause inflammation, triggering metabolic diseases like high blood pressure, heart attack, stroke, and diabetes.Despite the clear health implications of body fat distribution, relatively little is known about the genetic basis of body shape. A large international study that appeared in Nature in February began to fill in this gap by looking for regions of the human genome associated with a common metric known as the waist-to-hip ratio, which uses waist measurements as a proxy for visceral fat and hip measurements as a proxy for subcutaneous fat. The researchers analyzed samples from 224,000 people and found dozens of hot spots linked to their waist-hip ratio, including a few near a gene called Plexin D1 which is known to be involved in building blood vessels. Rawls and his postdoctoral fellow James E. Minchin, Ph.D., were curious about how a gene for growing blood vessels might control the storage and shape of fat cells. When they knocked out the Plexin D1 gene in mice, all of the mutant animals died at birth. So they turned to another model organism, the zebrafish, to conduct the rest of their experiments. Because these small aquarium fish are transparent for much of their lives, the researchers could directly visualize how fat was distributed differently between animals that had been genetically engineered to lack Plexin D1 and those with the gene still intact.By using a chemical dye that fluorescently stained all fat cells, the researchers could see that the mutant zebrafish had less visceral fat than their normal counterparts. They also noticed that the shape or morphology of the fat cells themselves was different. The zebrafish without the Plexin D1 gene had visceral fat tissue that was composed of smaller, but more numerous cells, a characteristic known to decrease the risk of insulin resistance and metabolic disease in humans. In contrast, their normal siblings had visceral fat tissue containing larger, but fewer fat cells of the kind known to be more likely to leak inflammatory substances that contribute to illness.
To determine how these findings related to metabolic disease, Minchin put the zebrafish on a high-fat diet. After a few weeks of adding egg yolks to their typical chow, Minchin found that the differences in fat distribution between the mutant and the normal zebrafish became even more pronounced. He then gave the fish a glucose tolerance test to see how their bodies responded to sugar. The mutants did a better job of clearing sugar out of their bloodstream and seemed to be protected from developing insulin resistance, a risk factor for diabetes and heart disease.Bolstering the zebrafish findings, collaborators at the Karolinska Institute in Sweden analyzed human patient samples and showed that levels of Plexin D1 were higher in individuals with type 2 diabetes, suggesting it may play a similar role in humans.“We think that Plexin D1 is functioning within blood vessels to pattern the environment in visceral fat tissue," said Minchin, who was lead author of the study. That is, the genes that build blood vessels are also setting up structures to house fat cells. "And this role skews the distribution and shape of fat in one direction or another,” he said. “It is probably just one of many of different genes that each contribute to overall body shape and metabolic health.” The researchers are actively searching for other genes as well as environmental factors that are involved in the biology of body fat, again using zebrafish models. “Our results indicate that the genetic architecture of body fat distribution is shared between fish and humans, which represents about 450 million years of evolutionary divergence," Rawls said. "For these pathways to have been conserved for so long suggests that they are serving an important role.”The research was supported in part by grants from the National Institutes of Health (DK081426, DK091356, DK093399, HL092263, R01HL118768), UNC UCRF Pilot Research Project Award, a Pew Scholars in Biomedical Sciences Award, and American Heart Association Postdoctoral Fellowships (11POST7360004, 13POST1690097).CITATION: "Plexin D1 determines body fat distribution by regulating the type V collagen microenvironment in visceral adipose tissue," James E.N. Minchin, Ingrid Dahlman, Christopher J. Harvey, Niklas Mejhert, Manvendra K. Singh, Jonathan A. Epstein, Jesús Torres-Vázquez, and John F. Rawls. Proceedings of the National Academy of Sciences, March 23, 2015. DOI: 10.1073/pnas.1416412112